Salud Mental 2015;
ISSN: 0185-3325
DOI: 10.17711/SM.0185-3325.2015.041
Received first version: April 2, 2014. Second version: January 13, 2015. Accepted: March 4, 2015.
Reward and aversion systems of the brain as a functional unit. Basic mechanisms and functions.
Anaclara Michel Chávez 1 , Bruno Estañol Vidal 1 , Horacio Sentíes Madrid 1 , Erwin Chiquete 1 , Guillermo R Delgado 1 , Guillermina Castillo Maya 1
1 Department of Neurology and Psychiatry, Laboratory of Clinical Neurophysiology, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City.
Correspondence: Anaclara Michel-Chávez. Vasco de Quiroga 15, Tlalpan, 14000, Mexico DF. Tel: (55) 5568 - 3450. E-mail: bestanol@hotmail.com
Abstract
Introduction. It is increasingly important to recognize the reward and aversion systems of the brain as a functional unit. A fundamental task of the mammalian brain is to assign an emotional/motivational valence to any stimuli by determining whether they are rewarding and should be approached or are aversive and should be avoided. Internal stimuli are also assigned an emotional/motivational valence in a similar fashion.
Objective. To understand the basic mechanisms and functions of the reward and aversion system of the brain.
Method. A bibliographical search was conducted in the Pubmed database using different key words. Documents on relevant aspects of the topic were selected.
Results. In the ventral tegmental area, dopaminergic (VTA-DA) neurons play a role in reward-dependent behaviors. It is also known that the inhibition of the VTA-DA neurons by GABAergic neurons contributes to a reward prediction error calculation that promotes behaviors associated with aversion. The ventral dopaminergic mesolimbic system and the nucleus accumbens are activated during reward and inhibited during aversions. The amygdala is activated during aversive behavior.
Discussion and conclusion. The reward/aversion system is highly relevant for survival, which is most likely its primary function. It is involved in important pathologies such as addiction, depression and autonomic and endocrine disturbances. Therefore, its knowledge has become of clinical importance.
Although great advances have been made in the knowledge of the basic mechanisms of the reward/aversion system, the detailed circuits within the VTA that mediate reward and aversion and the anatomical substrates are not completely clear.
Key words: Reward system, aversion system, dopaminergic systems, limbic system, pleasure.
Resumen
Introducción. Es muy importante reconocer el sistema de recompensa y aversión del cerebro como una unidad funcional. Una de las funciones fundamentales del cerebro de los mamíferos es la capacidad para designar un valor emocional/motivacional a cualquier estímulo. Esta capacidad permite identificar un estímulo como gratificante y aproximarnos a él, o reconocerlo como aversivo y evitarlo.
Objetivo. Comprender los mecanismos fisiológicos del sistema de recompensa-aversión.
Método. Se realizó una búsqueda bibliográfica en la base de datos Pubmed con las diferentes palabras clave. Se seleccionaron los documentos sobre los aspectos relevantes.
Resultados. Las neuronas dopaminérgicas del área tegmental ventral (ATV) cumplen un papel importante en los comportamientos dependientes de la recompensa. Asimismo, la inhibición de las neuronas dopaminérgicas ATV por parte de las neuronas GABAérgicas contribuye a predecir la recompensa y promueve comportamientos aversivos. Este sistema se activa durante actividades de recompensa y se inhibe durante la aversión. La amígdala es la principal estructura relacionada con la aversión.
Discusión y conclusión. Este sistema se considera de gran importancia para la supervivencia de las especies, la que parece ser su función primordial. Interviene en distintas patologías como adicciones, depresión, trastorno por estrés postraumático, fobias y trastornos endocrinos y autonómicos, por lo que el conocimiento de este sistema es de gran importancia clínica.
Aunque se ha avanzado mucho en el estudio y entendimiento de este sistema y de sus circuitos anatómicos ubicados en el ATV mesencefálica y sus conexiones con áreas subcorticales, el conocimiento de este sistema funcional sigue siendo un desafío científico.
Palabras clave: Sistema de recompensa, sistema de aversión, sistemas dopaminérgicos, sistema límbico, placer.
Anatomy and generalities
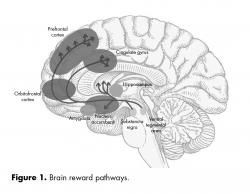
The limbic system is an old brain structure that plays an important role in learning and memory functions. It is also involved in the generation, integration and control of emotions and their behavioral responses. For instance, the interpretation of facial expressions and the underlying emotional status of a person, or the evaluation of a dangerous situation and the decision to express an appropriate behavioral response (e.g. “fight or flight”), involve a variety of limbic brain regions, including the amygdala and prefrontal cortical regions. The limbic system is also tightly linked to the autonomic nervous system and, via the hypothalamus, regulates endocrine, cardiovascular and visceral functions. The amygdala, hippocampus and medial prefrontal cortex (PFC) critically influence the responses of the hypothalamic-pituitary-adrenal axis (HPAA).1,2 Furthermore, dysfunctions in these brain areas are implicated in the etiology of mental disorders, including depression and posttraumatic stress disorder, which are often characterized by HPAA abnormalities,3 and in attention deficit-hyperactivity disorder.2,4
The amygdala is connected to the PFC, hippocampus, septum and dorsomedial thalamus. Because of its connectivity within the limbic system, it plays an important role in the mediation and control of emotions, including love and affection, fear, aggression, and reward, and therefore is essential for social behavior and the survival of the individual and the survival of the species. For instance, damage to the amygdala reduces aggressive behavior and the experience of fear (i.e, makes one less fearful), whereas electrical stimulation of the amygdala has the opposite effect.1,2
The nucleus accumbens plays an important role in reward, pleasure, emotions, aggression and fear. The core and shell subregions of the nucleus accumbens receive inputs from a variety of limbic and prefrontal regions, including the amygdala and hippocampus, and make up a network involved in acquisition, encoding and retrieval of aversive learning and memory processes.5
The PFC can be roughly subdivided into: dorsolateral, medial (which may include the functionally related anterior cingulate cortex) and orbitofrontal cortex (OFC).6 Both the medial PFC and OFC are part of a frontostriatal circuit that has strong connections to the amygdala and other limbic regions.7 Thus, prefrontal regions are anatomically well suited to integrate cognitive and emotional functions.8
The OFC is involved in sensory integration and higher cognitive functions, including decision making. The OFC also plays an essential role in a variety of emotional functions, such as judgment of the hedonic aspects of rewards or reinforcers, which are important for the planning of behavioral responses associated with reward and punishment. Dysfunction or damage to the OFC can result in poor empathy and impaired social interactions. The OFC also plays a major role in the regulation of aggression and impulsivity, and damage to the OFC leads to behavioral disinhibition, such as compulsive gambling, drug use and violence.8
The cingulate cortex can be cytoarchitectonically and functionally differentiated into an anterior part, which exerts executive functions, and a posterior part, which is evaluative. Furthermore, the cingulate cortex has two major subdivisions: a dorsal cognitive division and a rostral-ventral affective division.9 A variety of studies indicate an anatomic and functional continuum rather than strictly segregated operations.9,10
The ventral and dorsal areas of the cingulate cortex play a role in autonomic and a variety of rational cognitive functions, such as reward anticipation, decision making, empathy, and emotional regulation 11 (figure 1).
Reward and aversion
In 1954, James Olds and Peter Milner reported the results of what became a milestone in the research on reward mechanisms. They inserted electrodes into the brains of rats and then placed the rats in operant chambers equipped with a lever that, when depressed, would deliver current to the electrodes. Under these conditions, when an electrode was implanted in certain regions of the brain, notably the septal area, the rats would press the lever “to stimulate [themselves] in these places frequently and regularly for long periods of time if permitted to do so”.12 Not only will animals work for food when they were hungry or for water when they were thirsty, but even when sated rats would work for electrical stimulation of their brains.12,13
The seminal work of Olds and Milner unleashed a barrage of research studies into the brain mechanisms of reward, most immediately, a spate of studies on electrical brain reward.13
The biological basis of mood-related states, such as reward and aversion, is not yet fully understood, albeit it is recognized as a functional neuronal network. Classical formulations of these states implicate the mesocorticolimbic system, comprising brain areas that include the nucleus accumbens (NAc), the ventral tegmental area (VTA) and the prefrontal cortex, implicated reward;14,15,16,17 and the amygdala (AMG), periaquaductal gray (PAG), and the locus coeruleus (LC), often implicated in aversion.14,18
However, the notion that certain brain areas narrowly and rigidly mediate reward or aversion are becoming archaic in regard with the development of sophisticated tools and methodologies that provide new evidence.17
The NAc, especially its outer region, called the shell, has been shown to play a necessary role in assigning motivational properties on rewards and to the stimuli that predict them. Thus, for example, in rat and mouse models, an intact NAc is required if the animal is to learn to work (e.g. lever pressing) to obtain natural rewards, such as palatable foods, or to learn how to self-administer drugs. Once an animal learns how to obtain a reward, and the relevant behaviors become ingrained, reward seeking no longer depends on the NAc and is supported by the dorsal striatum (the caudate and putamen in humans), the brain structure that underlies well-learned behaviors and habits.7,19
Under natural conditions, speed and efficiency in gaining food, water and shelter improve the probability of survival. Thus, a critical role of reward circuitry is to facilitate the rapid learning of cues that predict the proximity of reward and of the behaviors that maximize the chances of successfully obtaining it. Once learned, predictive cues automatically activate cognitive, physiologic and behavioral responses aimed at obtaining the predicted reward.20,21
VTA dopamine neurons are the primary source of dopamine (DA) in target structures such as the medial prefrontal cortex and the NAc, which play important roles in a broad range of motivating behaviors and neuropsychiatric disorders.22,23,24
Dopamine, released from VTA neurons in the NAc, plays the key role in binding rewards and reward associated cues to adaptive reward-seeking responses.25,26 In animals, implanted electrodes can record the firing of dopamine neurons; microdialysis catheters and electrochemical methods can be used to detect dopamine.27 In humans, positron emission tomography (PET) permits indirect measures of dopamine release by observing the displacement of a positron-emitting D2 dopamine receptor ligand previously bound to receptors following a stimulus or pharmacologic challenge. Using such methods in multiple paradigms, it has been well-established that natural rewards cause firing of neurons and dopamine release in the NAc and other forebrain regions. When dopamine action is blocked −whether by lesioning dopamine neurons, blocking post-synaptic dopamine receptors or inhibiting dopamine synthesis− rewards no longer motivate the behaviors necessary to obtain them.27,28,29
Much evidence suggests that the precise pattern of dopamine neuron firing, and the resulting synaptic release of dopamine in forebrain circuits, act to shape behavior so as to maximize future reward.19,30
In a basal state, dopamine neurons have a slow tonic pattern of firing. When a new, unexpected, or greater than expected reward is encountered, there is a phasic burst of firing of dopamine neurons causing a transient increase in synaptic dopamine. When a reward is predicted from known cues and is exactly as expected, there is no change from the tonic pattern of firing, that is, there is no additional dopamine release. When a predicted reward is omitted or less than expected, dopamine neurons pause their firing to levels below their tonic rate. Phasic increases in synaptic dopamine signify that the world is better than expected, facilitate learning of new predictive information and bind the newly learned predictive cues to action.20,21
Two distinct patterns of dopamine neural activity occur in the behaving animal. Midbrain dopamine neurons typically fire at low frequencies of 1-5 Hz, which are thought to produce a tone on the high-affinity dopamine D2 receptor in terminal regions of the mesolimbic dopamine system, including the NAc. In contrast, when animals are presented with motivationally salient stimuli, such as conditioned cues that predict drug availability, midbrain dopamine neurons fire in high frequency bursts (>20 Hz), thereby producing transient increases in NAc DA concentrations that are sufficiently to occupy low affinity DA D1 receptors.31,32,33
The pleasure–reward system
Reward involves multiple neuropsychological components: 1. the hedonic sensation of pleasure itself; 2. the motivation to obtain the reward (incentive component); and 3. the reward-related learning.34
Pleasure represents the subjective hedonistic value of rewards; it can indeed be either rewarding when it follows satisfaction, or incentive when it reinforces behaviors.35,36
Pleasure is not merely a sensation. Even a sensory pleasure such as a sweet taste requires the co-recruitment of additional specialized pleasure-generating neural circuitry to add the positive hedonic impact to the sweetness that elicits liking reactions.34,37
Different rewarding stimuli may elicit qualitatively and quantitatively different reward values. The pleasure or rewarding value associated with sexual intercourse may not only exceed in magnitude that associated with scratching an itch, but the two pleasures or rewarding values may themselves be in addition qualitatively distinct.13
There is evidence, in animals, of an increased dopaminergic activity in the VTA either from an unexpected reward or, after recognition of the reward characteristics, from the anticipation of the reward. Therefore, anticipation of a satisfaction activates neurochemical pleasure mechanisms and reinforces the obtaining behavior. In this way, pleasure contributes to an increased level of organism excitation.36
Endocannabinoid system
The endogenous cannabinoid system is a signaling system composed of cannabinoid receptors, endogenous ligands for these receptors and proteins involved in the formation and deactivation of these endogenous ligands.38,39,40,41
Two types of CB receptors have been identified. CB1 is highly expressed in the brain and mediate most of the psychoactive/central effects of cannabis10,10,42,43,44 Discovered later, CB2 are presented predominantly in the periphery and, at low levels, in some areas of the brain.43,44,45,46,47 In the dopaminergic mesolimbic system, the best known circuit involved in motivational processes, average to high concentrations of CB1 receptors are found in the terminal region, the striatum, whereas low concentrations of CB1 receptors are found in the origin, the ventral tegmental area (VTA).10,48
Neurobiology of addiction
All drugs of abuse increase dopamine concentrations in terminal regions of the mesolimbic dopamine system. Increases in NAc DA are theorized to mediate the primary positive reinforcing and rewarding properties of all known drugs of abuse.49,50,51,52
In addition, when animals are presented with conditioned stimuli that predict drug availability, transient dopamine events that are theorized to mediate the secondary reinforcing effects of drugs of abuse and initiate drug seeking are also observed in the NAc.31,53
Specifically, each drug mimics or enhances the actions of neurotransmitters at receptors for these neurotransmitters. Opioids are presumed to be habit-forming because of actions at opiate receptors, and so is nicotine because of the action at nicotinic acetylcholine receptors. Phencyclidine acts at N-methyl-D-aspartate (NMDA) and sigma receptors, and also blocks dopamine reuptake.5,54 Tetrahydrocannabinol (THC) binds to endocannabinoid receptors.55,56 Although amphetamines and cocaine do not act directly at dopamine receptors, they are reinforcing because they increase the concentration of dopamine at the dopamine receptors of the nucleus accumbens and frontal cortex.16
It has become clear that the acute administration of most drugs of abuse increases dopamine transmission in the basal ganglia,16 and that dopamine transmission in this brain region plays a crucial role in mediating the reinforcing effects of these drugs.57,58,59,60
The mesolimbic dopamine pathway is made up of dopaminergic cells in the VTA projecting into the NAc, located in the ventral striatum, and is considered crucial for drug reward.54 The mesostriatal (dopamine cells of the substantia nigra projecting to the dorsal striatum) and mesocortical (dopamine cells of the VTA projecting to the frontal cortex) pathways are also recognized in contributing to predicting drug reward (anticipation) and addiction.61 The time course of dopamine signaling is also a key factor, where the fastest time course predominantly has a role in reward and attributing value to predicted outcomes of behavior, while steady activation of dopamine release plays a role in providing an enabling effect on specific behavior-related systems.24 The mode of dopamine cell firing (phasic vs. tonic) also differently modulates the rewarding, conditioning effects of drugs (predominantly phasic) versus the changes in executive function that occur in addiction (predominantly tonic) 62 (table 1).
The aversion system by the amygdala
Pavlovian fear conditioning is an associative learning task in which subjects are presented with a neutral conditioned stimulus (CS) paired with an innately aversive unconditioned stimulus (US).63
Pavlovian fear conditioning (and its aversive reinforcement) is severely impaired by disruption of the amygdala, indicating that the amygdala plays an important role in learning to respond defensively to stimuli that predict aversion.63,64
Fear is an intervening variable between sets of context-dependent stimuli and suites of behavioral response.
Unlike with reflexes, in the case of an emotion like fear, this link is much more flexible and the state can exist prior to and after the eliciting stimuli.65,66
Previous studies have shown that electrical stimulation of the amygdala evokes fear-associated responses such as cardiovascular changes,67,68 potentiated startle,69 and freezing.70
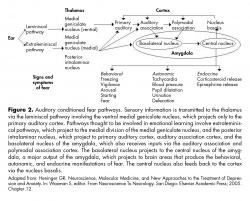
Contemporary fear models posit that the basolateral amygdalar (basal and lateral nuclei) complex is interconnected with the central nucleus (CeA), which is thought to be the main amygdaloid output structure sending efferent fibers to various autonomic and somatomotor centers involved in mediating specific fear responses.71
Neural activity in the amygdala appears to be required for expression of both conditioned and unconditioned responses to aversive stimuli, whereas synaptic plasticity appears to be required only for acquisition of conditioned responses, and not for expression of conditioned or unconditioned responses.63
After fear memory consolidation, which requires 4–6 h, fear memory becomes remarkably resistant to perturbation, giving way only to numerous unreinforced CS presentations, which lead to the extinction of learned fear responses. However, substantial remnants of the originally learned fear survive even after extensive extinction and causes the reappearance of fear-related behavior in a variety of circumstances, such as fear renewal and facilitated reacquisition.72
The lateral amygdala is essential in the acquisition and consolidation of auditory cued-fear conditioning. Thalamic and cortical auditory inputs to the LA are potentiated after fear conditioning, which are relayed to the basal and central amygdala so as to evoke aversive behavior.72
It seems that the fear-conditioning circuitry of the amygdala is functionally lateralized according to which side of the body (left or right) a predicted threat is anticipated on.73
The LA neuronal population displayed increased average CS-evoked firing after conditioning, decreased responses after extinction, and potentiates responses after reconditioning, in tight correlation with the changes in the freezing responses 72,74 (figure 2).
Conclusion
The reward and aversion systems are important mechanisms for individual and species survival (which is present in all mammalian animals and perhaps in all animals). The mesolimbic cortical dopaminergic system is the main substrate for the reward system, and the main neurotransmitter is dopamine, although serotonin, noradrenaline, endogenous opiates and cannabinoids, and perhaps other transmitters, also play a role.
The original function of the reward/aversion system is most likely the survival of the individual and the species, by promoting defense and/or pleasure mechanisms. However, the reward system is also important for sexual behavior, nursing, eating and drinking behavior. Potent psychoactive drugs stimulate in a non-natural way (massive stimuli) the reward/aversion system causing a disproportionate and unnatural response, and thereby inducing addiction and sensitization.
The aversive system may help the animal inducing a fight/flight behavior or avoiding some food. The amygdala is probably the main anatomical structure subserving aversive behavior, although the inhibition of the reward systems may also trigger aversive behavior.
Activation and sensitization (tachyphilaxia) of the aversive system is likely to be involved in anxiety disorders, panic attacks, post-traumatic stress disorders, phobias and abstinence syndromes
The reward/aversion system is closely related to memory, emotions and the autonomic and endocrine systems, and it is also related to cognitive functions through its connections to the prefrontal lobe. There is a heuristic value in considering the reward/aversion system as a functional unit. Both systems are crucial for survival and this common function should not be underestimated, particularly from an evolutionary viewpoint. Both systems share, albeit not all, anatomical structures and many neurotransmitters substances. It is perhaps an oversimplification to state that the withdrawal of one system leads to the overaction of the other but this hypothesis should be explored in the future in clinical and animal research.
Funding
None.
Conflict of interest
No author of this paper has a conflict of interest, including specific financial interest, relationships, and/or affiliations relevant to the subject matter included in this manuscript.
REFERENCIAS
1. Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci 2007;30:259–288.
2. Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry 2005;29:1201–1213.
3. Phillips PE, Stuber GD, Heien ML, Wightman RM et al. Subsecond dopamine release promotes cocaine seeking. Nature 2003;422:614–618.
4. Happaney K, Zelazo PD, Stuss DT. Development of orbitofrontal function: current themes and future directions. Brain Cogn 2004;55:1–10.
5. Mohanty A, Engels S, Herrington JD et al. Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology 2007;44:343–351.
6. Hyman SE. Chapter 30: Biology of addiction. En: Goldman L, Schafer AI (24 Ed.) Goldman´s Cecil medicine. Philadelphia, PA: Elseviere Saunder; 2012.
7. Tsou K, Brown S, Sanudo-Pena MC, Mackie K et al. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 1998;83:393–411.
8. Dreyer JK, Herrik KF, Berg RW, Hounsgaard JD. Influence of phasic and tonic dopamine release on receptor activation. J Neurosci 2010;30:14273–14283.
9. Sesack SR, Grace AA. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacol 2010;35:27–47.
10. Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Tr Neurosci 2007;30:194–202.
11. Bush G. Cingulate, frontal, and parietal cortical dysfunction in attention-deficit/ hyperactivity disorder. Biol Psychiatry 2011;69(12):1160–1167.
12. Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol 1954;47(6):419-427.
13. Marks LE. A brief history of sensation and reward. In: Gottfried JA (ed.). Neurobiology of sensation and reward. Boca Raton (FL): Chapter 2: CRC Press; 2011.
14. McEwen BS, Magarinos AM. Stress effects on morphology and function of the hippocampus. Ann N Y Acad Sci 1997;821:271–284.
15. William C, Mark, T. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology 2009;56(Suppl 1):122–132.
16. Grace AA. The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction 2000;95(Suppl 2):S119–S128.
17. Robinson TE, Berridge KC. Addiction. Annu Rev Psychol 2003;54:25–53.
18. Fride E. Endocannabinoids in the central nervous system: from neuronal networks to behavior. Curr Drug Targets CNS Neurol Disord 2005;4:633–642.
19. Oleson EB, Cheer, JF. A brain on cannabinoids: The role of dopamine release in reward seeking. Cold Spring Harb Perspect Med 2012;2:a012229.
20. Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature 2009;459:837–841.
21. Cohen JY, Haesler S, Vong L, Lowell BB et al. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature 2012;482:85–88.
22. Bock J, Braun K. The impact of perinatal stress on the functional maturation of prefronto-cortical synaptic circuits: implications for the pathophysiology of ADHD? Prog Brain Res 2011;189:155–169.
23. Carlezon WA Jr, Wise RA. Rewarding actions of phencyclidine and related drugs in nucleus accumbens shell and frontal cortex. J Neurosci 1996;16(9):3112–3122.
24. Onaivi ES, Ishiguro H, Gong JP, Patel S Et al. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci 2006;1074:514–536.
25. Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 2000;4(6):215–222.
26. Urban NB, Martínez, D. Neurobiology of addiction. Insight from neurochemical imaging. Psychiatr Clin N Am 2012;35:521–541.
27. Roberts DC, Corcoran ME, Fibiger HC. On the role of ascending catecholaminergic systems in intravenous self- administration of cocaine. Pharmacol Biochem Behav 1977;6:615–620.
28. Kim Y, Wood J, Moghaddam B. Coordinated activity of ventral tegmental neurons adapts to appetitive and aversive learning. PLoS ONE 2012;7(1):e29766.
29. Vogel Z, Barg J, Levy R, Saya D et al. Anandamide, a brain endogenous compound, interacts specifically with cannabinoid receptors and inhibits adenylate cyclase. J Neurochem 1993;61(1):352–355.
30. Wise RA. The role of reward pathways in the development of drug dependence. Pharmacol Ther 1987;35(1–2):227–263.
31. Solinas M, Goldberg SR, Piomelli D. The endocannabinoid system in brain reward processes. British J Pharmacology 2008;154:369–383.
32. Bozarth MA, Wise R. Intracranial self-administration of morphine into the ventral tegmental area in rats. Life Sci 1981;28:551–555.
34. Berridge KC, Kringelbach ML. Neuroscience of affect: Brain mechanisms of pleasure and displeasure. Curr Opin Neurobiol 2013;23(3):294–303.
35. Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat Rev Neurosci 2008;9(4):314-320.
36. Chenu A, Tassin JP. Pleasure: Neurobiological conception and Freudian conception. Encephale 2014;40(2):100-107.
37. Berridge KC. Pleasures of the brain. Brain Cogn 2003;52(1):106-128.
38. Wise RA, Rompré PP. Brain dopamine and reward. Annu Rev Psychol 1989;40:191–225.
39. Herkenham M, Lynn AB, Johnson MR, Melvin LS et al. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 1991;11:563–583.
40. Braun K. The brefrontal-limbic system: Development, neuroanatomy, function, and implications for socioemotional development. Clin Perinatol 2011;38:685–702.
41. Goeders NE, Smith JE. Cortical dopaminergic involvement in cocaine reinforcement. Science 1983;221:773–775.
42. Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to selfadministration of cocaine. Science 1987;237:1219–1223.
43. Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 2010;68:815–834.
44. Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: A hypothesis for the etiology of schizophrenia. Neuroscience 1991;41:1–24.
45. Devane WA, Axelrod J. Enzymatic synthesis of anandamide, an endogenous ligand for the cannabinoid receptor, by brain membranes. Proc Natl Acad Sci U S A 1994;91(14):6698–6701.
46. Phillips AG, LePiane G. Disruption of conditioned taste aversion in the rat by stimulation of amygdale: a conditioning effect, not amnesia. J Comp Physiol Psychol 1980;94:664–674.
47. Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 2003;4:873–884.
48. Owesson-White CA, Ariansen J, Stuber GD, Cleaveland NA et al. Neural encoding of cocaine-seeking behavior is coincident with phasic dopamine release in the accumbens core and shell. Eur J Neurosci 2009;30:1117–1127.
49. Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993;365:61–65.
50. Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 2005;310:329–332.
51. Wise RA. Roles for nigrostriatal—not just mesocorticolimbic—dopamine in reward and addiction. Trends Neurosci 2009;32(10):517–524.
52. Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev 2003;83:1017–1066.
53. Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov 2004;3:771–784.
54. Witten IB, Steinberg EE, Lee SY, Davidson TJ et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron 2011;72:721–733.
55. Herkenham M, Lynn AB, Little MD, Johnson MR et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA 87:1932–1936.
56. Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 2001;24(2):97–129.
57. Van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA neurons disrupts reward consumption. Neuron 2012;73:1184–1194.
58. Gong JP, Onaivi ES, Ishiguro H, Liu QR et al. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res 2006;1071:10–23.
59. Lammel S, Lim BK, Ran C, Huang KW et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature 2012;491(7423):212–217.
60. Blair HT, Sotres-Bayon F, Moita MA, Ledoux JE. The Lateral Amygdala Processes The Value of Conditioned and Unconditioned Aversive Stimuli. Neuroscience 2005;133(2):561–569.
61. Koob GF. Neural mechanisms of drug reinforcement. Ann N Y Acad Sci 1992;654:171–191.
62. Hyman SE, Malenka RC, Nestlr EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 2006;29:565-598.
63. Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J Comp Physiol Psychol 1972;81:281–290.
64. Rosen JB, Davis M. Enhancement of acoustic startle by electrical stimulation of the amygdala. Behav Neurosci 1988;102(2):195–202.
65. Wise RA, Bozarth MA. Brain mechanisms of drug reward and euphoria. Psychiatr Med 1985;3:445–460.
66. Applegate CD, Kapp BS, Underwood MD, McNall CL. Autonomic and somatomotor effects of amygdala central N. stimulation in awake rabbits. Physiol Behav 1983;31(3):353–360.
67. Weingarten H, White N. Exploration evoked by electrical stimulation of the amygdala of rats. Physiol Psychol 1978;6(2):229–235.
68. Joo Kima E, Horovitzb O, Pellmana B, Mimi Tana L et al. Dorsal periaqueductal gray-amygdala pathway conveys both innate and learned fear responses in rats. PNAS 2013;110(36):14795–14800.
69. Bobae An, Hong I, Choi S. Long-term neural correlates of reversible fear learning in the lateral amygdala. J Neuroscience 2012;32(47):16845–16856.
70. Blair H, Huynh V, Vaz V, Van J et al. Unilateral storage of fear memories by the amygdala. J Neuroscience 2005;25(16):4198–4205.
71. Iwata J, Chida K, LeDoux JE. Cardiovascular responses elicited by stimulation of neurons in the central amygdaloid nucleus in awake but not anesthetized rats resemble conditioned emotional responses. Brain Res 1987;418(1):183–188.
72. Tan KR, Yvon C, Turiault M, Mirzabekov JJ Et al. GABA neurons of the VTA drive conditioned place aversion. Neuron 2012;73:1173–1183.
73. Adolphs, R. The biology of fear. Curr Biol 2013;23(2):R79–R93.
74. Heninger GR. Neuroscience, molecular medicine, and new approaches to the treatment of depression and anxiety. En: Waxman S (ed.). From neuroscience to neurology. San Diego: Elsevier Academic Press; 2005.